KIPA provides full GMP support to achieve PICsGMP/EU GMP or equivalent GMP accreditation

The well-known and respected KIPA team has a proven track record in full GMP (GxP) compliance projects in several ASEAN countries, Far East and South Asia.
KIPA professional GMP training and consulting expertise also attract Health Ministries and government organizations in the ASEAN region.


Our clients achieved PICsGMP/EU GMP or equivalent GMP accreditation within planned timelines, initially set budgets and high-value export business ever since towards regulated markets and within ASEAN region.
Whether you have an API or finished product plant and you see your target growth in export to regulated markets you have to contact the best GMP consulting partner. Contact us and find out how best we can suit your target. We can support you in achieving high performance.
Our portfolio of GMP service:
|
|
 |
Basic Principles of GMPLine opening:
|
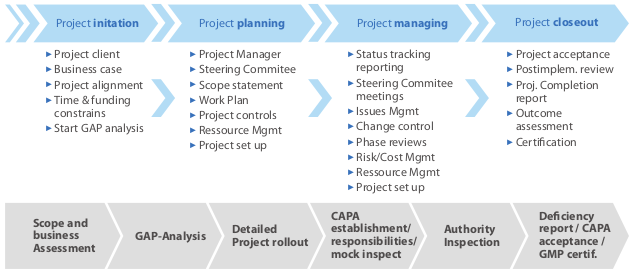
KIPA project methodology chart:
|
|
ITIL®/ITSM, ISEC, GAMP and CSV consulting by KIPA
Authority inspectors tend to increasingly incorporate full GxP compliance in their inspections. In the past, most of these authorities did not put their emphasis on auditing computerized systems as the majority of state-of-the-art pharmaceutical manufacturers have all production related and product release proof established in a well documented QM system. Modern drug manufacturing, however, relies heavily on computerized systems. That is why ensuring quality and accuracy of data is absolutely crucial and that is also why Computerized Systems Validation (CSV) is necessary to assure that critical processes are functioning properly to assure a high quality of the product. During several international GxP inspections, we experienced the enhanced capabilities of authority inspectors who are well-trained in the inspection of such IT systems in order to verify the GxP compliance status also for large-volume and high-tech operating pharmaceutical manufacturers.
Computerized System Validation
CSV is the documented process of assuring that a computer system does exactly what it is designed for in a consistent and reproducible manner. The validation process begins with the system proposal and requirements definition and continues throughout the system‘s whole life cycle up to system retirement and retention of the e-records based on the applicable regulatory rules.
This includes all companies with:
- computerized production processes and/or (GMP)
- computerized release relevant activities in QC (GLP)
- computerized warehouse handling and/or automated shipping (GDP)
- or any other IT-based manufacturing, laboratory, clinical, distribution procedure
Companies that have computerized activities with key criticality to the final product but cannot provide these GxP relevant basics will face critical problems during EU/WHO/TGA etc. GMP inspections now and in future.
We have incorporated such requirements in our consulting support concept and are proud to have been appointed in some ASEAN countries as head consultants for CSV compliance review, consultant for establishment of IT quality documentation and consultant for establishment of a complete IT security concept.
|
For a successful GMP inspection followed by the corresponding GMP certification
|
We are offering the full range of compliance support:
|

